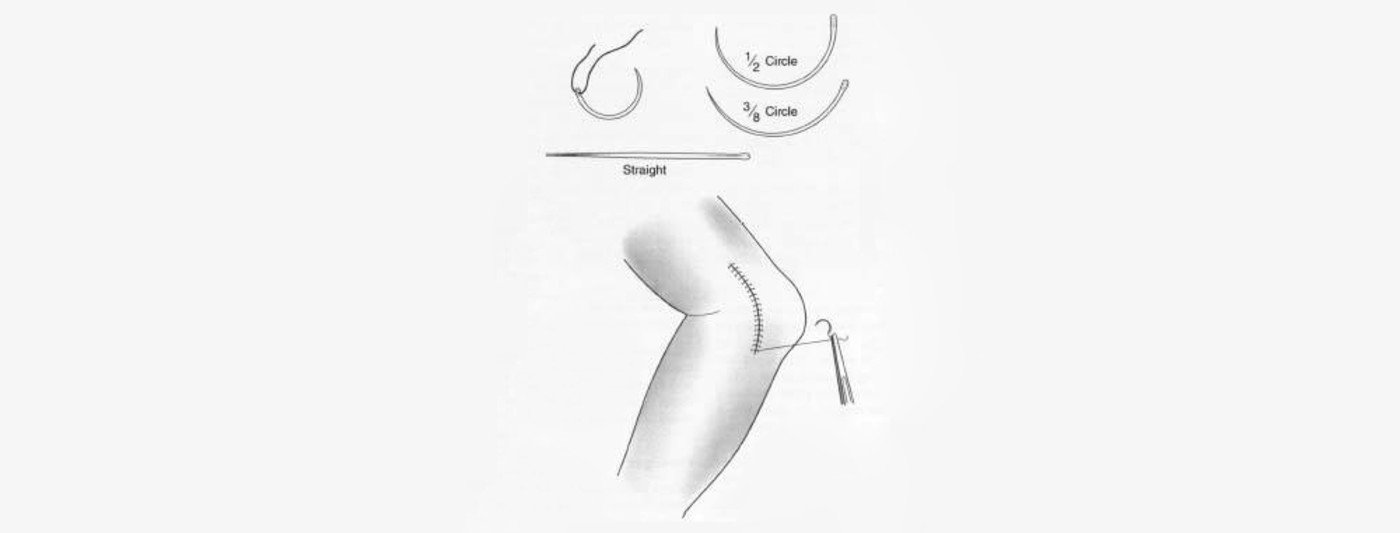
Sutures are also classified according to their form. Some are monofilaments, that is, consisting of only one thread-like structure. Others consist of several filaments braided or twisted together. Surgeons choose which type of suture to use depending on the operation. A monofilament has what is called low tissue drag, meaning it passes smoothly through tissue. Braided or twisted sutures may have higher tissue drag, but are easier to knot and have greater knot strength. Braided sutures are usually coated to improve tissue drag. Other sutures may have a braided or twisted core within a smooth sleeve of extruded material. These are known as pseudo-monofilaments. A suture can also be classified according to its diameter. In the United States, suture diameter is represented on a scale descending from 10 to 1, and then descending again from 1-0 to 12-0.
Suture manufacturing comes under the regulatory control of the Food and Drug Administration (FDA) because sutures are classified as medical devices. Manufacturing guidelines and testing for the industry is provided by a non-profit, non-governmental agency called United States Pharmacopeia, located in Rockville, Maryland.
Suture manufacturing comes under the regulatory control of the Food and Drug Administration (FDA) because sutures are classified as medical devices. Manufacturing guidelines and testing for the industry is provided by a non-profit, non-governmental agency called United States Pharmacopeia, located in Rockville, Maryland.